Sample Collection Room
Comfortable private booths for blood draws and specimen collection, managed by trained phlebotomists.

Our laboratory is equipped with advanced automated systems ensuring accuracy, reliability, and fast turnaround times. All equipment undergoes routine calibration and quality checks to maintain international diagnostic standards.
High-throughput automated machine providing accurate biochemical reports with minimal manual handling.
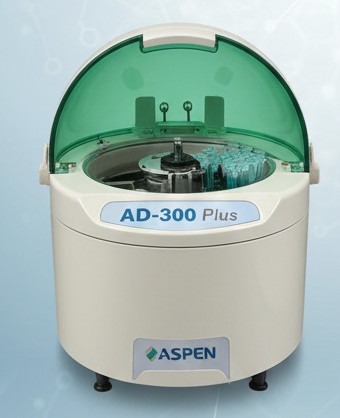
Measures essential electrolytes like Sodium, Potassium, and Chloride, vital for emergency and routine testing.

Specialized for thyroid, fertility, diabetes markers, and hormonal profiling with improved accuracy.

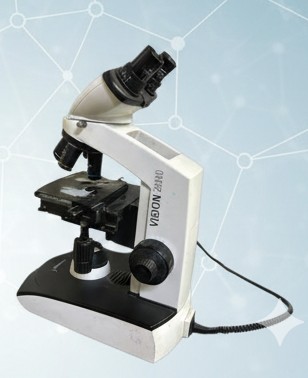
High-resolution optical microscope used for urine microscopy, blood cell morphology, and pathology screening.
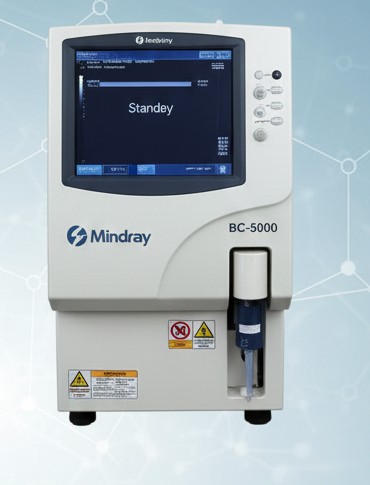
Advanced analyzer delivering CBC and blood cell counts with high accuracy, essential for diagnosis and monitoring.
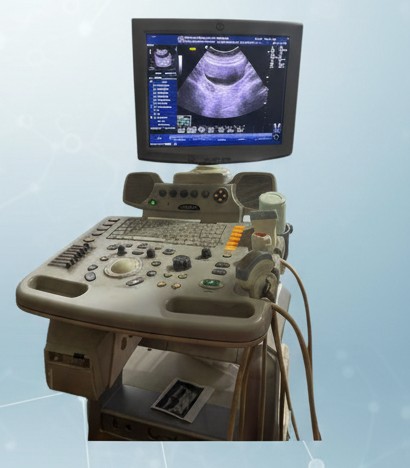
High-performance ultrasound system offering clear imaging for abdominal, obstetric, vascular, and general diagnostic scanning.
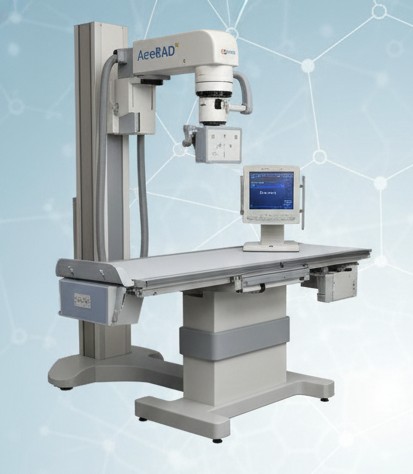
Advanced digital imaging system providing high-clarity X-ray scans with faster processing for accurate diagnosis and reduced radiation exposure.
Comprehensive facilities to collect, handle and store specimens with full traceability and biosafety.
Comfortable private booths for blood draws and specimen collection, managed by trained phlebotomists.
Dedicated area for labeling, centrifugation and aliquoting following strict SOPs to reduce errors.
Barcode labels scanned at every processing stage ensuring full chain-of-custody and traceability.
Temperature-monitored refrigerators with alarms and backup power for sensitive samples and reagents.
Secure long-term storage for special samples with continuous temperature monitoring and access control.
Validated cold-chain carriers ensuring safe movement between collection points and analysis areas.
Robust systems to ensure results you can trust.
Daily IQC runs with documented Levey-Jennings charts and corrective actions for out-of-range controls.
Active participation in CMC Vellore–approved EQAS programs to benchmark test accuracy, validate analytical performance, and maintain national quality standards.
Scheduled calibrations using certified reference standards with digital recordkeeping for audits.
Comprehensive SOP library covering all analytical workflows, reviewed and updated regularly.
Organized divisions enabling focused expertise and faster turnaround.
Safety-first practices to protect patients, staff and samples.
Color-coded segregation, safe storage and disposal through authorized waste handlers.
Mandatory PPE and periodic biosafety training for all laboratory personnel.
Air-conditioned and humidity-controlled lab spaces to ensure accuracy and instrument stability.
Use of sterile consumables and laminar flow cabinets for contamination-free processing.
Fire extinguishers, emergency exits, backup power and routine safety drills.
Restricted entry to sensitive areas and continuous surveillance for added security.
Digital systems that increase accuracy, speed and transparency.
Integrated LIS for ordering, tracking, reporting and audit-trail management.
Instant report generation with downloadable PDFs and authenticated signatures.
Easy online scheduling with e-consent, history tracking and patient login access.
Typical reporting times — may vary based on sample and workload.
| Test / Service | Typical TAT | Notes |
|---|---|---|
| CBC | 2 hours | Peripheral smear if required |
| Thyroid Profile | Same day | 6–8 hours depending on sample time |
| Ultrasound | 20 minutes | Report after radiologist confirmation |
| Digital X-Ray | 10 minutes | Report within 1–2 hours |
| Biochemistry Panels | 4–8 hours | Critical values notified urgently |
| Culture & Sensitivity | 48–72 hours | Based on organism growth |
| PCR / Molecular Tests | 24–48 hours | Varies by protocol |